Research Interests
Development of an original nano-delivery platform for improving the oral absorption of poorly absorbed drugs
Taher Nassar, Suha Attili-Qadry, Ayala Rohald; Natasha Nayarkin and Simon Benita
Based on combined technologies the aim of this novel nanoplatform is to release and enhance the intestinal absorption of drugs by encapsulating them in nanocapsules that are embedded in microparticles. This nanoplatform was shown to bypass the liver where part of the drug dose is degraded prior to reaching the blood circulation following oral administration since the drug-loaded nanocapsules permeated through the intestinal mucosa and reached the blood circulation via the lymphatic system.
This nanotechnology was successfully applied to various drugs. Docetaxel, a drug prescribed for the treatment of prostate, gastric, lung and breast cancer, is extensively metabolized in the gut wall and only administered intravenously, often causing adverse effects such as unpredictable acute hypersensitivity. With an original oral formulation of docetaxel nanocapsules embedded in microparticles yielded in rats, we demonstrated a higher bioavailability compared with the intravenous administration of the commercial product. These findings were further confirmed in mini-pigs. Furthermore; improved anticancer activity compared with intravenous Taxotere®, was observed in the metastatic lung cancer model in severe Combined Immune Deficiency beige (SCID-bg) mice.

Targeted delivery of chemotherapeutic drugs to specific cancerous cells using nanoparticles conjugated to monoclonal antibodies
Taher Nassar; Nour Karra and Simon Benita
Cancer is one of the most lethal and the leading cause of death worldwide. chemotherapy provides only partial success in cancer treatments. Consequently, efforts are concentrated on the development of improved treatment strategies, mainly site-specific therapies. Owing to the over-expression of specific surface antigens on cancer cells, particular focus has been directed towards the active targeting of nano-sized drug delivery systems, especially biocompatible polymeric nanoparticles (NPs) via their conjugation to targeting ligands. The up-regulated expression of the epidermal growth factor receptor (EGFR) is one of the most prominent changes observed in solid tumors including lung cancer, providing a strong rationale to develop EGFR targeted therapies. To target EGFR over-expressed in lung cancer, cetuximab-conjugated nanoparticles were designed and loaded with the lipophilic paclitaxel palmitate (pcpl) prodrug using a novel cross-linker molecule, oleyl cysteineamide synthesized in our laboratory. We demonstrated the in vitro targeting efficiency and improved cellular internalization and cytotoxicity of this targeted delivery system in lung cancer cells over-expressing EGFR. We reported in vivo tolerability and enhanced efficacy of cetuximab pcpl NPs in a metastatic lung cancer model. The overall results indicated the potential of this promising targeted platform for the improved treatment of lung cancer and other EGFR positive tumors.

| | 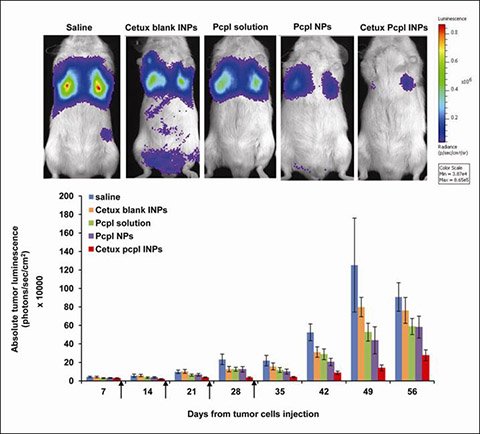 |
 | | Efficacy study. (A) Longitudinal detection and quantification of A549-luc-C8 tumor growth in live mice by the luciferase assay using a CCCD camera (N=6–9 animals per group). Tumor size is designated by the total luminescence intensity of the dorsal and ventral images, expressed in radiance units (photons/sec/cm2). Arrows indicate the treatment schedules on the 8th, 15th, 22nd and 29th day. Representative ventral images of mice taken on day 42 following tumor inoculation are given. Results are presented as average ± SEM. *P<0.05. In repeated measures ANOVA, significant differences were found within subjects depending on the time of tumor volume evaluation (Greenhouse-Geisser test) and significant differences between subject effects depended on the treatment group. Scheffe's post hoc proved cetuximab pcpl INPs to be significantly more efficient in tumor inhibition over time than all other treatments. Isolated pairwise comparisons revealed no differences between saline and cetuximab BLK INPs (p>0.05), whereas a significant difference was noted betweem pcpl NPs and cetuximab pcpl INPs in tumor inhibition (p<0.05). All statistical differences were found significant both when analysis was performed on all animals (until day 49) and on those that survived until day 56. The total luminescence in the saline-treated control begins to "decrease" from day 49, as some animals in this group were already euthanized, and the ones remaining were those which initially had a lower tumor burden. Since animals fulfilling euthanasia criteria were euthanized from day 49 onwards, imaging was discontinued on day 56 but body weight and survival were recorded until the last surviving mouse (i.e. end of study). |
Morphological evaluation of immunonanoparticles and respective surface-activated controls. Images A-D: TEM images of uranyl acetate negatively stained oleyl cysteineamide surface-activated PLGA blank NPs (A-B) and cetuximab blank INPs (C-D) at different areas of the grid and at various magnifications. E-F: AFM phase plot images of oleyl cysteineamide-activated NPs (E) and cetuximab INPs (F) (scanning area size of 2µm). G-H: Cryo-TEM images of cetuximab INPs (G) and oleyl cysteineamide-activated NPs (H). | | |
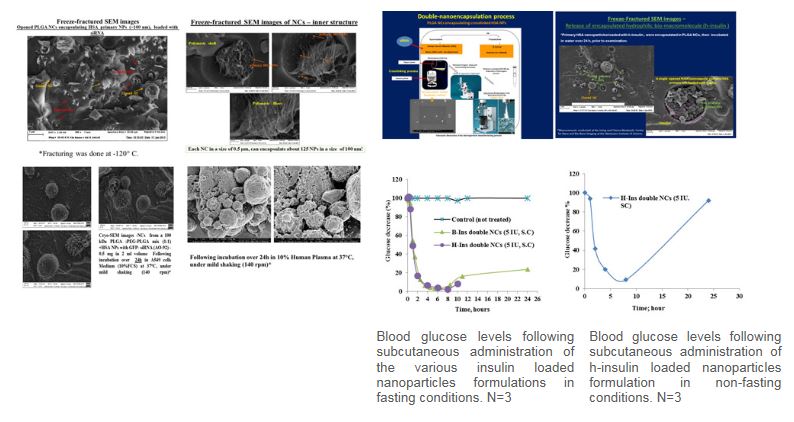
Protection and controlled release of peptides, proteins, oligonucleotides and siRNA using a double nanotechnology platform
Taher Nassar, Orit Amsalem, Simon Benita
Hydrophilic bio-macromolecules, such as peptides, proteins, siRNA and antisense oligonucleotides (AO), are characterized by poor membrane permeability and high sensitivity to environmental degradation conditions (heat, pH, enzymes) affecting their effective delivery and the exploitation of their therapeutic potential. The successful intracellular delivery of these biomacromolecules is a key determinant of their therapeutic effectiveness. Thus, there is still a clear incentive to develop novel delivery strategies that will improve treatment outcomes with biomacromolecules. This research program proposes a novel formulation of double nano-encapsulated of bio-macromolecules using a primary nanoparticle (NP) coated with a secondary submicron capsule (NC). This new method, developed in our lab, produced the final double nano vehicles in the form of dry powder while preserving the loaded integrity of siRNAs, peptides and proteins. This new double nano vehicle, consists of the biodegradable polymers; PLGA (Poly D,L-lactic-co-glycolic acid) and HSA (human serum albumin), both compatible for injectable administration in humans. This powder can be easily formulated into injectable dispersions.Preliminary in vitro and in vivo experiments are encouraging. If this original nano-delivery system, will be successful, it will offer pioneering therapeutic opportunities to the clinicians treating severe chronic diseases and genetic deficiencies, via a promising arsenal of novel controlled delivery systems, embedding sensitive, potent, and short acting biological drugs, for the benefit of the patients.
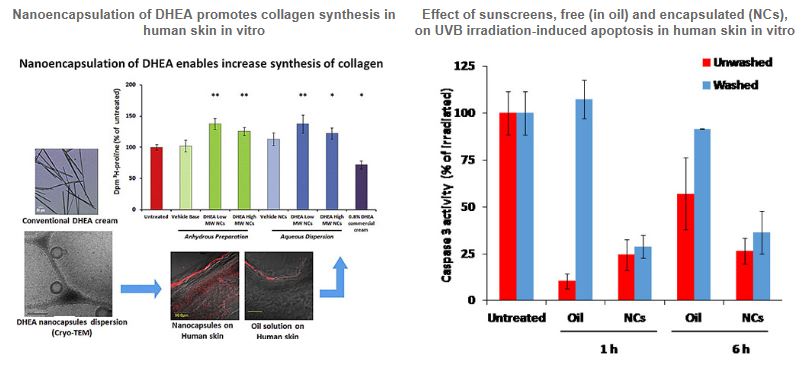
Nanospheres and Nanocapsules for Improved Performance of Skin Topical Treatments – Poorly Absorbed Drugs and Sunscreens
Amit Badihi, Eve Bohbot-Shoshani, Nir Debotton, Marina Frušić-Zlotkin, Yoram Soroka and Simon Benita
The skin represents a major challenge for topical application of therapeutic and cosmetic substances, mainly due to its unique biological structure, epidermal barrier, which limits the free diffusion of active substance through the stratum corneum (SC). The passive percutaneous permeation of poorly-absorbed active ingredients can be improved by adding specific enhancers to the formulation or by using adequate nanodelivery systems such as nanospheres (NSs) or nanocapsules (NCs). Our polymer-based nanocarriers offer a versatile and safe topical applications route, which may be exploited by the skin natural structure towards improved cutaneous or even systemic bioavailability.
Our focus is on the relation between the levels of the active compounds penetrating the skin and the outcome of the biological activities. This was demonstrated by the enhanced accumulation of DHEA within the dermis following NCs application and the concomitant collagen synthesis increase, or by the protective effect on the skin of avobenzone NCs toward UVA/UVB irradiation.
If successful, the present study will add to the dermatology field original topical platforms that may provide a therapeutic tool of high clinical significance. Such achievements may improve the existing topical therapeutic protocols or even suggest novel strategies for skin disorder treatments.
| | | |
 | | |

