
I became fascinated by developmental biology as a second-year biology student, when I first watched a living sea urchin embryo develop under the microscope. At that moment I decided to pursue the understanding of developmental processes. In my PhD studies in the Technion Faculty of Medicine, I uncovered gene networks that act across tissues to generate the hindbrain in the vertebrate embryo and specify distinct cell types within it.
In my postdoctoral research in The University of Pennsylvania I have broadened my scope to understanding developmental processes at a cell biological level and in intra-cellular resolution.
The oocyte, the precursor cell of the egg, is the only cell that upon fertilization, can give rise to an entire organism. Oocytes undergo a fascinating differentiation process, simultaneously orchestrating dramatic nuclear events of meiosis, cytoplasmic polarization, dynamic changes in inter-cellular organization, and cellular growth.
I have discovered a novel cellular organizer that integrates multiple cellular processes and differentiation programs in the oocyte. This organizer is based on the oocyte centrosome and I termed it the Centrosome Organizing Center (COC).
My new lab in the Hebrew University investigates the functional and regulatory mechanisms of the COC in multiple stages of early oocyte differentiation. We are investigating how the COC is localized by mitotic cell divisions and activated by a specific cellular organization in the ovary, how it coordinates nuclear and cytoplasmic events, and how it regulates the cytoskeleton to simultaneously control meiotic chromosomal dynamics and cell polarity. We are also investigating how the COC regulates a prion-like protein in assembling a physiologically functional prion-like aggregate, and how the COC controls mechanical forces and cell synchrony by a novel primary cilium in the oocyte. In addition, we are studying the regulation of germline stem cells in the ovary in generating differentiating oocytes. This allows us to study how multiple differentiation programs are coordinated by the cell on a single platform of early oogenesis. Dissecting COC mechanisms will advance our understanding of their abrogation in different disease like cancer, prion-borne neurodegenerative disease, and ciliopathies, as well as fertility and reproduction conditions.
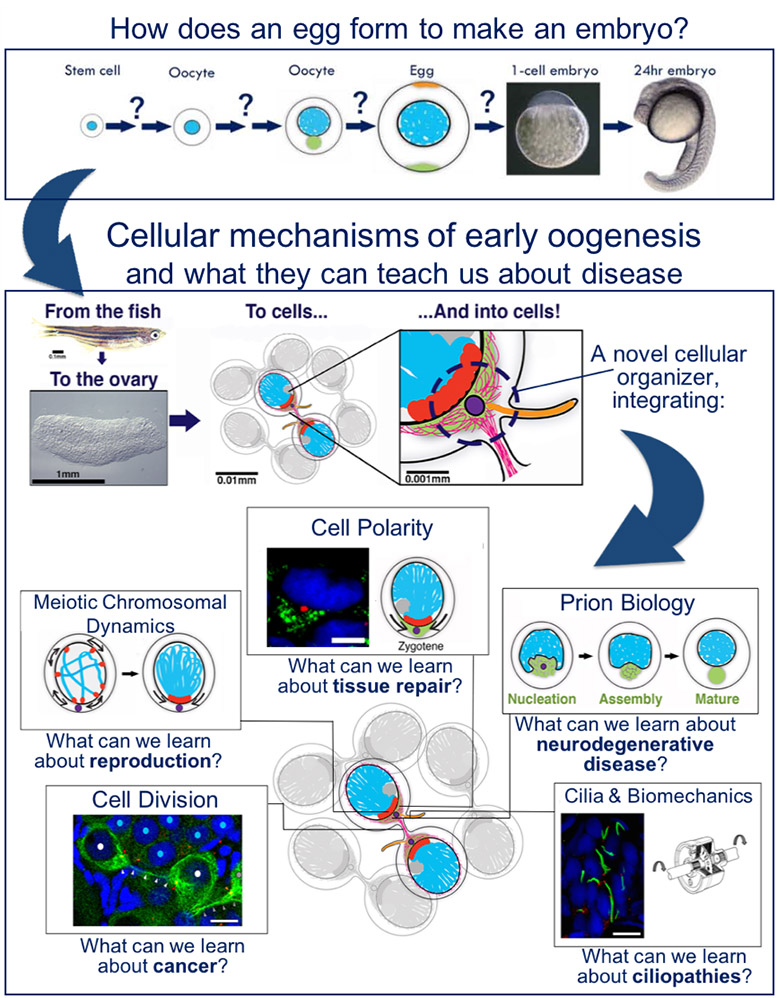
We use the zebrafish as our excellent animal model and utilize methods of advanced genetics and microscopy. My long-term goal is to construct comprehensive mechanistic models for early oogenesis in vivo that lead to the understanding of the cell biology of a differentiating cell generally, and are applicable to disease.

