Goal
We seek to better understand the molecular and cellular mechanisms that underlie normal development and congenital malformations of eyes and forebrain.
To achieve this goal, we use genetic and chemical manipulations of zebrafish embryos, which enable us to generate and study these processes in vivo in a vertebrate model organism.
Model system
Over the past 2.5 decades zebrafish have developed as an eminent model for embryonic development, physiology, disease modeling and drug discovery. The zebrafish embryo shows many similarities to other vertebrates and to mammals at various levels, including molecular, cellular and physiological.
Advantages of this model system include fast ex-utero development of embryos, amenability to genetic manipulations, and embryo transparency that allows long-term live imaging at very high resolution.
Methods
A large variety of methods is used in our studies and includes:
- Live imaging using advanced microscopy and transgenic embryos
- Tissue analysis using histology, in situ hybridization and immunohistochemistry
- Transcriptomics
- Drug screens
Research Projects:
Interactions between the eye and its
vasculature during development:
Ocular vascularization defects play a major
role in several human eye diseases that lead to impaired vision. Hence,
understanding the molecular mechanisms that govern normal and abnormal ocular
blood vessel development could provide important insights into the mechanisms
underlying these diseases.

We are studying two main questions:
1. Is
patterning of ocular vessels influenced by eye tissues? The early vasculature
of the zebrafish eye is patterned in a highly stereotypic manner. What signals
dictate the formation of this pattern? We use transgenic lines, live imaging
and chemical manipulations to address this question.
2. Do blood vessels contribute in a
non-metabolic way to development of eye tissues? Using several zebrafish
mutants and genomic methods we are investigating this intriguing question.
Zebrafish models for human eye
malformations and diseases:
We use genetic mutants with eye and lens
malformations as models for abnormal development of these organs. By
understanding the mechanisms leading to these malformations we can improve our
ability to predict congenital eye problems, and possibly to prevent eye
diseases

Six3: Mutations
in human SIX3 are associated with
holoprosencephaly (HPE), the most common forebrain malformation, often including
various eye malformations.
We developed a zebrafish model of Six3 loss of function in which embryos show optic disc coloboma, abnormal optic nerve development and deficiencies in the ventral midline of the forebrain. This model can now be used to further identify specific defects in the holoprosencephalic brain, and to better understand the roles of Six3 in eye morphogenesis and in the pathogenesis of HPE.

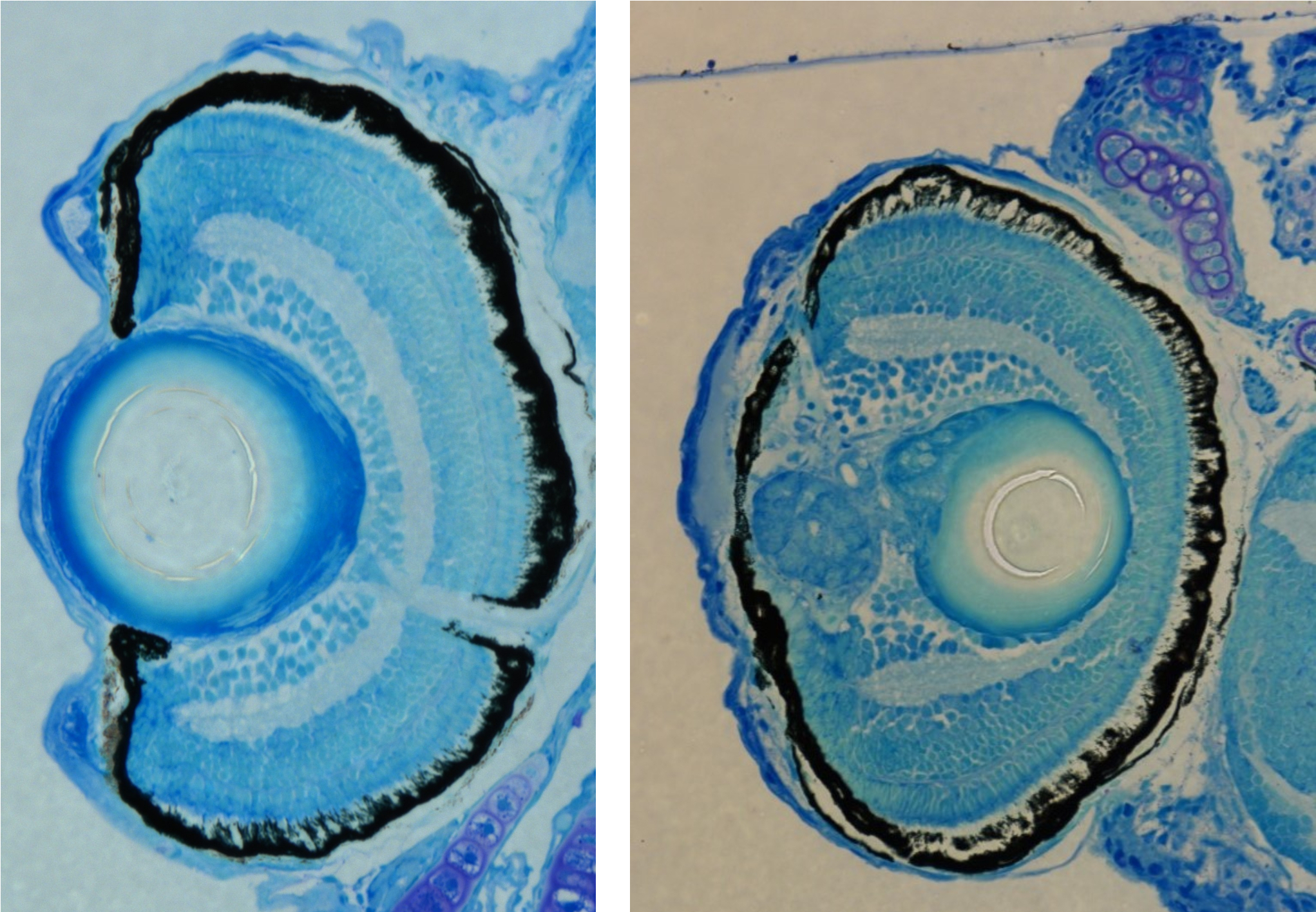
A model for secondary cataract:
Secondary cataract is the main complication of cataract surgeries,
millions of which are carried out every year worldwide. We have identified a
genetic mutation leading to development of lens abnormality with features of
secondary cataract, driven by excessive TGFb signaling. We use this new model to
screen for drugs that can interfere with pathological processes driven by TGFb signaling, not only in the lens but in other organs as well.

