Recent Articles
Myristoylation Confers Oral Bioavailability and Improves the Bioactivity of c(MyD 4−4), a Cyclic Peptide Inhibitor of MyD88
Published in: Shira Dishon; Adi Schumacher-Klinger; Chaim Gilon; Amnon Hoffman; Gabriel Nussbaum; Mol. Pharmaceutics 16, 1516-1522, DOI: 10.1021/acs.molpharmaceut.8b01180
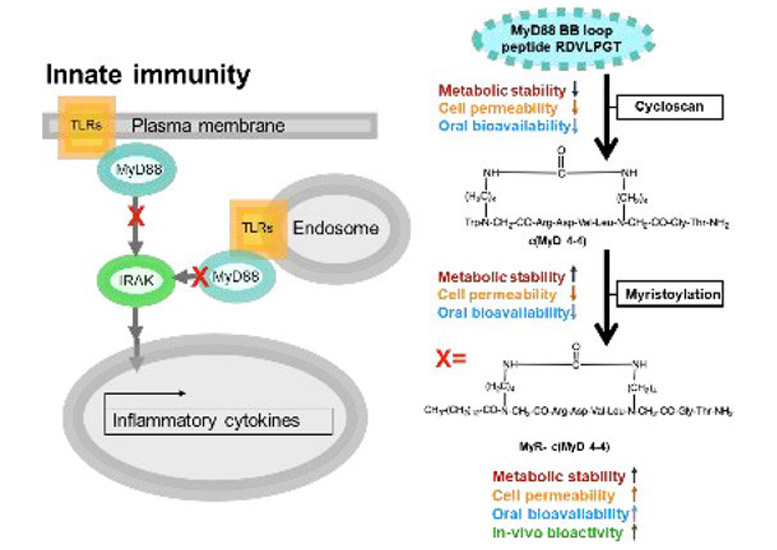
Myeloid differentiation primary response 88 (MyD88) is an intracellular adaptor protein central to the signaling of multiple receptors involved in inflammation. Since innate immune inflammation promotes autoimmunity, MyD88 is an attractive target in autoimmune disease. We previously developed c(MyD 4–4), a novel cyclic peptide competitive inhibitor of MyD88 dimerization that is metabolically stable. Parenteral administration of c(MyD 4–4) reduces disease severity in a mouse model of the human autoimmune disease multiple sclerosis. We now show that N-terminal myristoylation of c(MyD 4–4) enhances the competitive inhibition of MyD88 dimerization in living cells, leading to improved inhibition of the Toll-like receptor and IL-1 receptor signaling. Importantly, myristoylation converts c(MyD 4–4) to an orally bioavailable inhibitor of MyD88. Oral administration of c(MyD 4–4) significantly lowered the inflammatory cytokines secreted by peripheral autoimmune T cells in mice immunized with myelin antigens and ameliorated disease severity in the mouse model of multiple sclerosis. Taken together, we show the conversion of a protein active region to a metabolically stable, selective cyclic peptide that is orally bioavailable.
Orally Active Peptides: Is There a Magic Bullet?
Published in: Räder AFB, Weinmüller M, Reichart F, Schumacher-Klinger A, Merzbach S, Gilon C, Hoffman A, Kessler H. (2018) Angew Chem Int Ed Engl. 57(44):14414-14438.
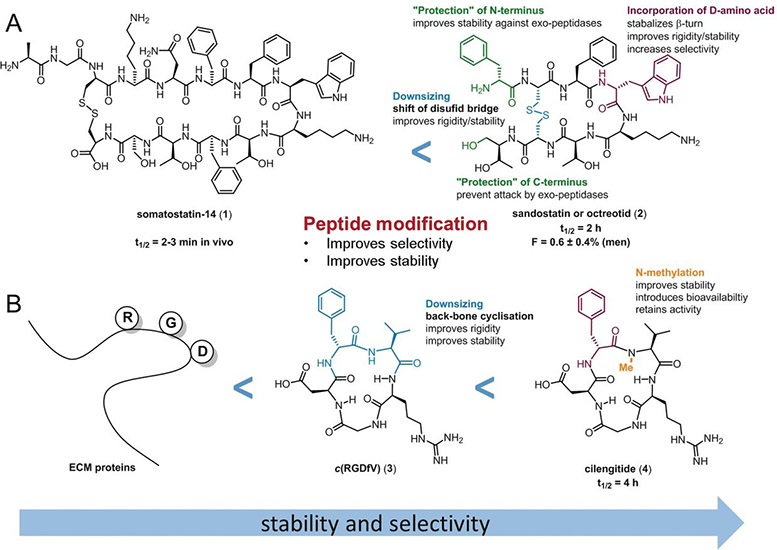
For decades, the development of peptides as potential drugs was aimed solely at peptides with the highest affinity, receptor selectivity, or stability against enzymatic degradation. However, optimization of their oral availability is highly desirable to establish orally active peptides as potential drug candidates for everyday use. A twofold optimization process is necessary to produce orally active peptides: 1) optimization of the affinity and selectivity and 2) optimization of the oral availability. These two steps must be performed sequentially for the rational design of orally active peptides. Nevertheless, additional knowledge is required to understand which structural changes increase oral availability, followed by incorporation of these elements into a peptide without changing its other biological properties. Considerable efforts have been made to understand the influence of these modifications on oral availability. One approach is to improve the oral availability of a peptide that has been previously optimized for biological activity, as described in (1) above. The second approach is to first identify an intestinally permeable, metabolically stable peptide scaffold and then introduce the functional groups necessary for the desired biological function. Previous approaches to achieving peptide oral availability have been claimed to have general applicability but, thus far, most of these solutions have not been successful in other cases. This Review discusses diverse chemical modifications, model peptides optimized for bioavailability, and orally active peptides to summarize the state of the research on the oral activity of peptides. We explain why no simple and straightforward strategy (i.e. a “magic bullet”) exists for the design of an orally active peptide with a druglike biological function.

