Our Research
Regulation of oxygen and reactive oxygen species homeostasis
Oxygen (O2) - we can't live without it but it is challenging to live with. On the one hand, O2 is vital for energy production in the powerhouse of the cell (mitochondria). This energy is essential for supporting the high energetic demand of multicellular organisms (including ourselves). On the other hand, the metabolism of O2 creates reactive oxygen species (ROS). ROS are extremely important for regulating many biological processes and have an essential role in immunity. However, too much ROS can induce oxidative injury and death. Indeed, non-regulated ROS production may underlie the development of many pathological conditions including atherosclerosis (hardening of the arteries), Alzheimer's and Parkinson's disease, and many age-associated diseases. Therefore, keeping the right level of O2 and ROS in tissues at all times (and this differs greatly between tissues!) is a major challenge for all aerobic animals, on which has to be resolved (a 24/7 job with no breaks!). However, the mechanisms that allow animals to coordinate O2 and ROS responses in different tissues and in response to changes in O2 level are still poorly understood.
To explore this, we use the nematode Caenorhabditis elegans (C. elegans) as a model system. The relative simplicity of the C. elegans nervous system, the vast genetic and imaging tools available, its simple and short life cycle, and the fact that it can behaviorally respond to changes in O2 levels make C. elegans the perfect model organism for this task. A short movie can be seen below that presents the slowing of worms' speed upon a 21% - 17% O2 shift. These worms express the GLB-5 neuroglobin gene that fine-tunes O2 responses in C. elegans.
Promoting healthspan by rejuvenating mitochondrial activity in old age
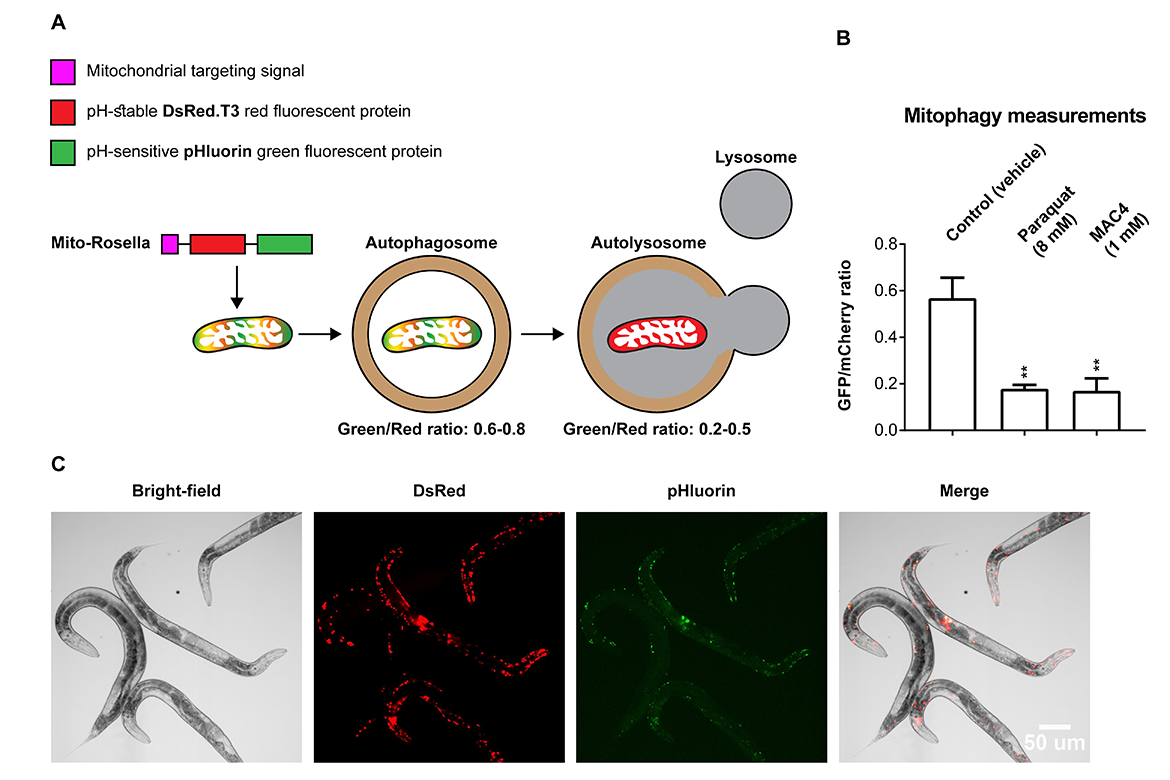
The most devastating aspect of aging is the debilitating morbidity associated with it. This is a result of a decline in the proper operation of a key quality-control system called autophagy, which in the case of mitochondrial-recycling, is called mitophagy. It ensures removal of damaged intracellular organelles and their replacement with newly formed ones. Age-associated malfunctioning result in morbidities such as Alzheimer's disease, Parkinson's disease, congestive heart failure and skeletal muscle weakness. Our laboratory has designed and synthesized novel mitophagy activating compounds (MACs). So far, we have promising results in several model systems including C. elegans, human cells, and primary rodent neurons. Below, a figure showing transgenic worms expressing the mitophagy genetically encoded sensor, i.e. MitoRosella, and a graph showing that MAC4 induces robust mitophagy.