Research Interests
Proteases are naturally expressed in all organisms and constitute 1-2% of the human genome; they play key roles in the regulation of several normal and pathological processes such as apoptosis, protein degradation, extracellular matrix turnover, antigen presentation and inflammation. Proteases are found to be misregulated in several pathologies such as cancer, atherosclerosis, arthritis and autoimmune diseases. All proteases are synthesized in the cell as inactive enzymes (zymogens). Their activation is tightly regulated post-translationally, consequently they are difficult to study since their abundance often does not correlate with their activity. My laboratory is interdisciplinary combining chemistry, biochemistry and pharmacology; our interests are in 1) design and synthesis of novel activity based probes (ABPs) that specifically target various cystein proteases. 2) Using these probes to investigate cystein protease function in various normal and pathological human processes and 3) applying fluorescently labeled ABPs for non-invasive imaging and tandem targeted therapy of various pathologies in vivo such as cancer and atherosclerosis. Most research projects in the lab combine chemical synthesis, cell biology, biochemistry, microscopy and in vivo imaging.
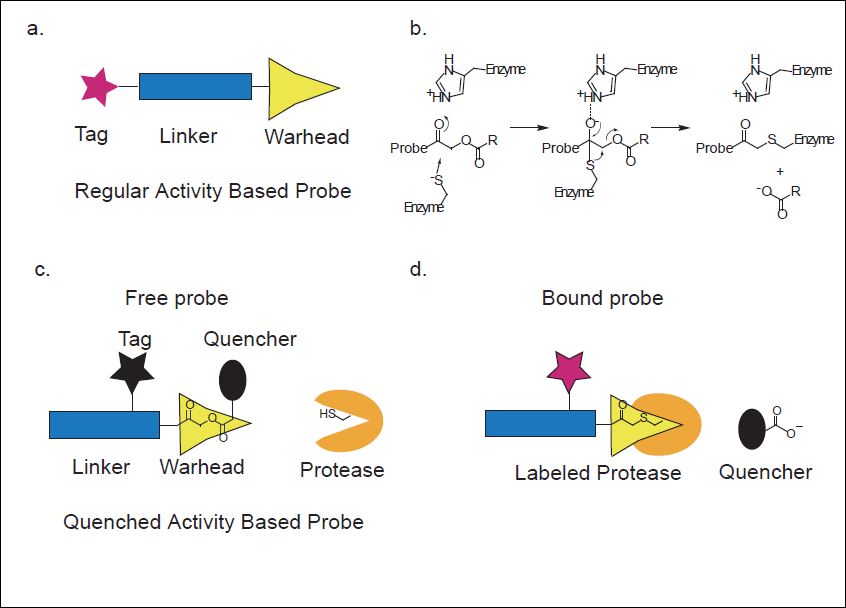
We are currently using synthetic chemistry methods for generating new probes that will be used for simultaneous non-invasive imaging and real time treatment of pathologies. These probes will be applied to mice models of cancer and atherosclerosis. In addition, we are designing novel fluorescent reagents that will target caspase activity in live cells and will allow for real time imaging of caspase activity during apoptosis. These probes will be further used to study chemotherapy resistance to cancer therapy. All in all we are convinced that our research will open new horizons in the fields of disease diagnostics combined with real time therapy.
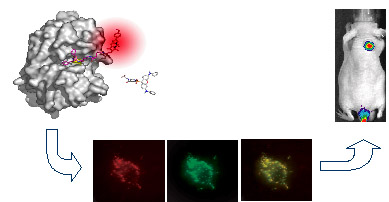
This scheme demonstrates the docking of a novel fluorescent probes to its
target cathepsin L; this unique probe generates a fluorescent signal only after
interaction with the active cysteine cathepsin. This probe was then applied to
real time imaging of cathepsin L in live cells. After chemical modification the
probe was applied for non-invasive fluorescent imaging of cancer in mice.

