Research
Introduction:
The anterior pituitary (AP) gland plays a central role in the physiology of the endocrine system. It controls important functions such as growth and metabolism, and regulates hormone secretion from other endocrine glands (e.g., thyroid gland, adrenal gland and gonads). Hypothalamic releasing hormones reach the AP via the hypothalamo hypophyseal portal system, and interact with specific receptors on AP cells to either stimulate or inhibit AP hormone section.
The AP gland contains five different types of hormone secreting cells that secrete at least seven different types of hormones. In our research, we focus on growth hormone (GH) secreting cells known as somatotrophs. GH is a Pleiotropic hormone that plays an essential role in somatic growth and metabolism, and affects aging and longevity. Two hypothalamic hormones primarily regulate GH secretion: GH-releasing hormone (GHRH) and somatostatin (SST), as well as the gastrointestinal tract hormone ghrelin. GHRH and Somatostatin exert their stimulatory and inhibitory effects, respectively, by activating two specific G-protein coupled receptors (GPCRs), GHRH receptors (GHRH-Rs) and somatostatin receptors (SST-Rs). Ghrelin exerts its stimulatory effects on GH secretion by activating GH secretagogue receptors (GHS-R). The cartoon in Figure 1 illustrates these three-receptor types.
AP somatotrophs are excitable cells. A plethora of voltage-gated Na+, K+, and Ca2+ channels generate either sporadic or rhythmic electrical activity in somatotrophs. The voltage-gated Ca2+ influx that is coupled to this electrical activity plays an essential role in the secretion of GH. GHRH and Somatostatin regulate GH secretion by regulating this voltage-gated Ca2+ influx. We therefore investigate the molecular properties of voltage-gated Ca2+ channels (VGCCs) in somatotrophs in relation to GH secretion.
Current Research:
We recently evaluated the relative contribution of various VGCCs to Ca2+ influx and to GH secretion in rat somatotrophs. We identified four types of high-voltage activated (HVA) Ca2+ channels in somatotrophs: L-type (Cav1.2 and Cav1.3), P/Q-type (Cav2.1), N-type (Cav2.2) and R-type (Cav2.3) (Figure 1). Ca2+ influx through each of these four Ca2+ channels could regulate GH secretion (Figure 2). However, L-type channels contribute the major component to HVA Ca2+ influx and to GH secretion (Figure 2). Interestingly, the HVA Ca2+ channels in somatotrophs are unevenly distributed on the surface membrane: L-type (Cav1.2, Cav1.3) and P/Q-type (Cav2.1) channels predominantly localize in lipid rafts whereas N-type (Cav2.2) and R-type (Cav2.3) channels predominantly localize in non-raft membrane domains (Figure1). This compartmentalization of Ca2+ channels among lipid raft and nonraft domains suggests a subcellular mechanism for differential regulation of Ca2+ influx by hypothalamic and peripheral hormones, under different physiological or pathological conditions.
Figure 1: Ca2+ channels in somatotrophs.
 The cartoon summarizes our current knowledge regarding the Ca2+ channel molecular machinery in rat pituitary somatotrophs. Illustrated are four types of HVA Ca2+ channels, LTCCs (Cav1.2, Cav1.3), P/Q-type (Cav2.1), N-type (Cav2.2) and R-type(Cav2.3). The HVA isoforms Cav1.2, Cav1.3 and Cav2.1 predominantly localize in lipid rafts (illustrated as the thick and red part of membrane). Additionally, the cartoon illustrates three types of TTCCs, Cav3.1 Cav3.2 Cav3.3, and three receptor types that regulate GH secretion, GHRH-R, SST-R and GHS-R (see text). LTCCs, L-type Ca2+ channels; TTCCs, T-type Ca2+ channels; HVA, high voltage-activated.
The cartoon summarizes our current knowledge regarding the Ca2+ channel molecular machinery in rat pituitary somatotrophs. Illustrated are four types of HVA Ca2+ channels, LTCCs (Cav1.2, Cav1.3), P/Q-type (Cav2.1), N-type (Cav2.2) and R-type(Cav2.3). The HVA isoforms Cav1.2, Cav1.3 and Cav2.1 predominantly localize in lipid rafts (illustrated as the thick and red part of membrane). Additionally, the cartoon illustrates three types of TTCCs, Cav3.1 Cav3.2 Cav3.3, and three receptor types that regulate GH secretion, GHRH-R, SST-R and GHS-R (see text). LTCCs, L-type Ca2+ channels; TTCCs, T-type Ca2+ channels; HVA, high voltage-activated.
Figure 2: Each of the HVA Ca2+ channels in somatotrophs can regulate GH secretion.
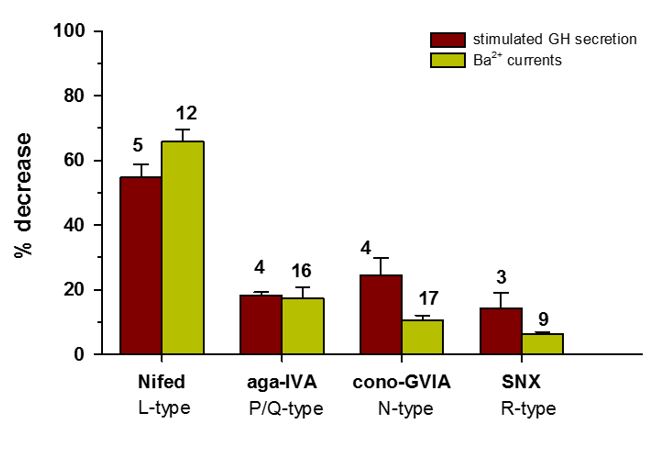 The Histogram compares between the effects of Ca2+ channel blockers on HVA Ba2+ influx and on "stimulated" GH secretion. The effects of Nifedipine (10 μM), ω-agatoxin-IVA (250 nM) and SNX-482 (30 nM) on L-, PQ- and R-type Ba2+ currents, respectively, were not significantly different from their effects on "stimulated" GH secretion. Yet, the effect of ω- conotoxin GVIA (2 μΜ) on N- type Ba2+ currents was significantly smaller than the effect on "stimulated" GH secretion. Nevertheless, the overall similarity between the effects of CCBs on HVA Ba2+ influx and on "stimulated" GH secretion demonstrates that each one of the four HVA Ca2+ channels in somatotrophs can regulate GH secretion.
The Histogram compares between the effects of Ca2+ channel blockers on HVA Ba2+ influx and on "stimulated" GH secretion. The effects of Nifedipine (10 μM), ω-agatoxin-IVA (250 nM) and SNX-482 (30 nM) on L-, PQ- and R-type Ba2+ currents, respectively, were not significantly different from their effects on "stimulated" GH secretion. Yet, the effect of ω- conotoxin GVIA (2 μΜ) on N- type Ba2+ currents was significantly smaller than the effect on "stimulated" GH secretion. Nevertheless, the overall similarity between the effects of CCBs on HVA Ba2+ influx and on "stimulated" GH secretion demonstrates that each one of the four HVA Ca2+ channels in somatotrophs can regulate GH secretion.
Lab Aims:
1. The molecular machinery of Ca2+ channels in pituitary cells: We identified five pore-forming α1-subunits (Cav-subunits) in somatotrophs (Cav1.2, Cav1.3, Cav2.1, Cav2.2 and Cav2.3). However, virtually nothing is known about the expression levels of these Cav isoforms, about the identity of their splice variants and about the identity of the Ca2+ channel auxiliary subunits (α2δ and β) in somatotrophs. Knowledge regarding the relative expression levels of these molecular components will identify the major Ca2+ channel complexes in somatotrophs and provide information about their pharmacological and physiological properties. From a therapeutic viewpoint, this information is essential for the development of pharmacological tools to treat pituitary disease, and for studying the potential involvement of Ca2+ channelopathies in pituitary disease.
2. Ca2+ channels as drug targets in the treatment of GH secretion disorders: L-type Ca2+ channels (LTCCs) are of special interest; they play a dominant role in Ca2+ influx and in GH secretion, and dihydropyridines (DHPs) block them effectively. This opens questions regarding the potential usage of DHP blockers in the treatment of GH oversecretion disorders such as Acromegaly and Gigantism.
3. The potential involvement of Ca2+ channelopathies in GH secretion disorders: An increasing number of studies in recent years have linked genetic missense mutations in LTCCs (CACNA1C, CACNA1D and CACNA1F) to diseases of the human cardiovascular, nervous and endocrine systems. These disease-associated genetic mutations occur at homologous functional positions (activation gates) in LTCCs. Thus, it is plausible that similar homologous missense mutations in somatotroph LTCCs (CACNA1C and CACNA1D) can cause abnormal GH secretion.

