Research Interests
Our lab
focuses on the design of novel therapies and innovative drug-delivery systems
by employing nanotechnology and advanced mass spectrometry imaging. We use
nanotechnology to maximize bioavailability, bioactivity and target organ
specificity of pharmaceuticals, and apply mass spectrometry imaging to monitor
dynamic interactions of drugs with their target organs, to study mechanistic
pathways of diseases, and to identify novel targets for therapeutic
intervention.
Nanotechnology
We
develop methods for modifying basic physicochemical properties of drugs in
order to assign desired characteristics to the final dosage form. We design
readily bioavailable nanometric drug delivery systems with improved biodistribution
properties for various delivery routes. We focus on addressing widespread
challenges associated with nanoparticle synthesis, e.g., uncontrollable
crystallization and crystal growth, particle aggregation and size increase,
loss of active pharmaceutical ingredient (API) activity, insufficient carrier
biocompatibility/biodegradability and more.
Mass
spectrometry imaging
We
employ mass spectrometry imaging (MSI) to study a wide variety of processes
occurring within any organ of interest as the result of various
pathophysiological processes and drug delivery. MSI is a powerful tool for
directly measuring the distribution of molecules in tissue sections. The uniqueness
of this technique is that it enables a high-resolution outlook on how various
substances distribute in organs at different time points without prior
knowledge of their existence or employing fluorescent or radioactive labels.
Hundreds of molecular species can be visualized within one single step, which
can reflect on the subtlest physiological processes triggered by pathological
conditions or external intervention such as drug delivery. Particularly we
strive to answer questions about metabolic changes accompanying disease
progression, as well as their response to drug treatment. We aim to identify
novel targets for therapeutic intervention, which can be crucial for disease
initiation and progression. In addition, we use MSI to monitor drug delivery
efficiency, such as tracking API release and distribution, revealing API and
its carrier co-localization, their impact on disease progression and
predisposing the target/adjacent organs to adverse effects, identification of
treatment-predictive biomarkers and stratification of drug delivery. We are
able to precisely map the changes in metabolite, lipid and peptide profiles,
and co-localize them with the delivered drug, its metabolites and the carrying
vehicle.
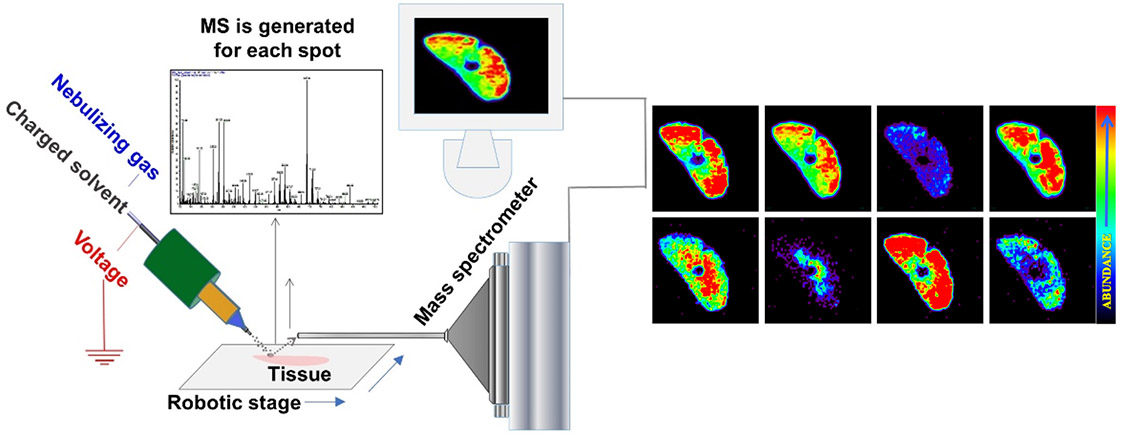
Fig.1. Schematic presentation of ambient mass spectrometry imaging technique - Desorption Electrospray Ionization Mass Spectrometry Imaging.
A beam of charged droplets is directed onto a tissue surface to desorb and ionize molecules, and the splash of these droplets carries the resultant ions into a mass spectrometer for analysis. A two-dimensional imaging stage moves the tissue at a controlled speed to record the mass spectra from different spatial coordinates and the signal is subsequently converted into images of molecular ion distributions. Each spectrum generates a single pixel in the image, whereas every mass peak can be translated into a separate color-coded map based on its intensity and the spatial distribution of its corresponding molecular ion.

