Lab Interests
Our laboratory studies the genetic mechanisms determining tumor growth and metastasis. In order for tumors to grow and to spread throughout the body, cancer cells must acquire various abnormal functions, and this occurs through changes in gene activity. We are interested in the functions acquired by cancer cells and in the genes controlling them.
Our research focuses on two major topics: the factors regulating the differentiation state of breast cancers, and the process of cellular senescence, a major roadblock to tumor development.
Molecular regulators of breast cancer differentiation state
Human tumors are highly heterogeneous, differing greatly in their characteristics and their severity. Breast cancers are classified into several subtypes, each displaying distinct traits: certain subtypes display rapid growth and metastasis, causing high mortality rates, while others are typically less aggressive. Highly aggressive tumors are often poorly differentiated, such that they have lost many of the characteristics displayed by the mature tissue and cells in it, and display similarities to normal mammary stem and progenitor cells.
Our work is aimed at uncovering the genetic regulators whose activity dictates poor differentiation of breast cancers. Our focus is on regulatory genes known to play a role in the control of normal stem cell function. Analysis of gene expression data derived from many tumors led us to identify previously unknown functions in breast cancer of several such stem cell-associated regulatory genes. One of these is TCF3 (TCF7L1), which encodes a transcription factor of the Wnt pathway-associated TCF/LEF family, and plays key roles in the regulation of epidermal and embryonic stem cell identity. We established that TCF3 is highly expressed in poorly differentiated, aggressive breast cancers and that it promotes the tumor initiation and growth capacity of breast cancer cells. Another gene of interest encodes the chromatin regulator EZH2, which plays an important role in controlling normal stem cell function as a component of the Polycomb complex. In breast cancers EZH2 acts to maintain the gene expression program associated with the highly aggressive and poorly differentiated basal-like subtype, including the expression of progenitor-associated genes. Moreover, EZH2 promotes the expansion of a 'bi-lineage' subpopulation of cells enriched for progenitor traits, which is uniquely found in this tumor type.
Our ongoing work is aimed at elucidating the regulatory transcriptional network controlling the differentiation state of these aggressive tumors and the action of specific factors in the context of this network.
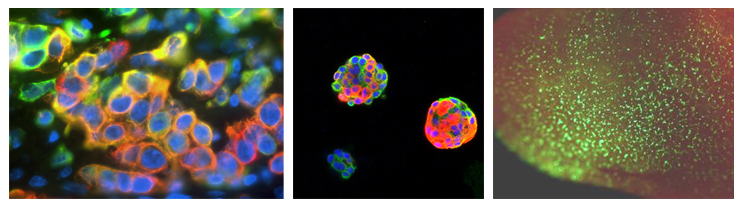
Left: Section of a human basal-like breast tumor stained for the lineage markers K14 (red) and K18 (green); bi-lineage cells appear orange. Center: Colonies formed by human breast cancer cells grown in 3D culture and stained for the same markers, indicating ongoing differentiation-like changes. Right: Mouse lung seeded with metastatic tumor cells, labeled with GFP (green).
Cellular senescence as a tumor-suppressive mechanism
In order to form a primary tumor mass cells must overcome barriers that block uncontrolled proliferation. Cellular senescence is a coordinated program that is activated by cells in response to a variety of physiologic stresses, and in response to oncogene activation. Senescent cells cease to proliferate and acquire a variety of distinct morphologic and metabolic traits. Senescence blocks the proliferation of damaged or potentially transformed cells, and is therefore a central tumor suppressive mechanism.
Our research is aimed at uncovering the functions of cellular senescence in vivo, in normal and cancerous tissues. Only limited understanding exists of the dynamics of senescent cell formation, the traits of senescent cells, the effects such cells exert on neighboring cells, and the mechanisms responsible for their elimination. We have developed mouse models that enable the induction of cellular senescence in a variety of normal tissues and in developing cancers. These mice allow us to study the process of senescent cell formation, aspects of senescent cell biology, the manner by which such cells affect tissue physiology, and the eventual fate of these cells.
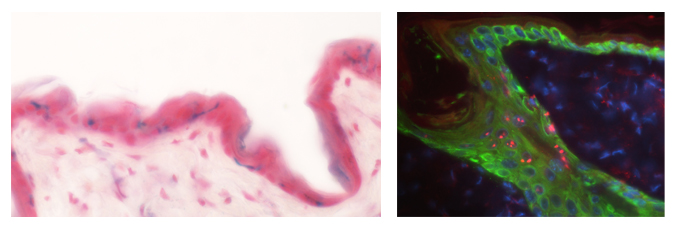
Left: Cellular senescence induced in the mouse skin, detected by SA-βGal stain (blue). Right: The p19ARF protein (red) is detected in senescent cells in the epidermis.

